Background: In the two largest phase 3 studies in patients with paroxysmal nocturnal hemoglobinuria (PNH), ravulizumab given every 8 weeks was noninferior to eculizumab given every 2 weeks across all efficacy endpoints. Data on efficacy and safety of ravulizumab in patients aged >65 years with PNH are limited.
Aims: To compare the efficacy and safety of ravulizumab in patients with PNH aged >65 years with those aged ≤65 years.
Methods: The population included patients from two phase 3 studies that assessed ravulizumab vs eculizumab in complement-inhibitor-naïve (301; NCT02946463) and -experienced (302; NCT03056040) adults with PNH. In study 301, patients were aged ≥18 years with a confirmed PNH diagnosis by flow cytometry and had a lactate dehydrogenase (LDH) level ≥1.5x upper limit of normal (ULN; 246U/L). In study 302, patients were aged ≥18 years with a confirmed PNH diagnosis by flow cytometry, were clinically stable on eculizumab having received ≥6 months of treatment and had a LDH level ≤1.5x ULN. Patients were randomized to either ravulizumab or eculizumab for 26 weeks after which all received ravulizumab up to 52 weeks. This prespecified analysis stratified patients by age: ≤65 or >65 years. Primary endpoints included percentage change in LDH from baseline to weeks 26 and 52, percentage of patients achieving LDH-normalization (LDH-N; LDH levels: ≤1x ULN) at weeks 26 and 52 and transfusion avoidance (TA) from baseline to weeks 26 and 52. Breakthrough hemolysis (BTH), hemoglobin (Hgb) stabilization and FACIT-fatigue score were secondary endpoints. Treatment emergent adverse events (TEAEs) were assessed as an indicator of safety.
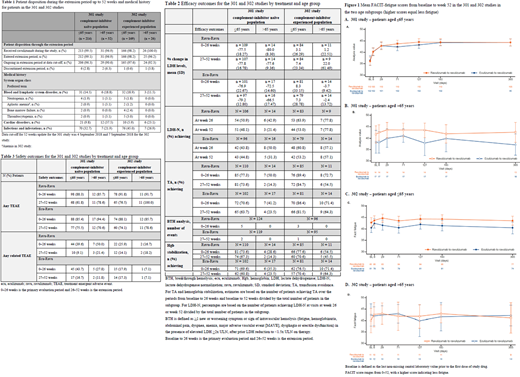
Results: A total of 58 patients aged >65 years and 383 patients aged ≤65 years were included. Disposition and medical history were similar among subgroups at baseline (Table 1). Results for primary and secondary endpoints for the two subgroups were comparable across studies and efficacy was maintained through 52 weeks. A higher proportion of treatment-experienced patients (>65 years) achieved all endpoints vs -naïve patients (Table 2). The percentage change in LDH levels from baseline to 26 and 52 weeks was similar between subgroups in study 301 (-66.5 to -80.0%) whereas in study 302, LDH levels remained stable in all subgroups up to 52 weeks (-3.7 to 22%). The percentage of patients achieving LDH-N in both studies at 26 and 52 weeks differed; 43.8-63.9% of patients aged ≤65 years achieved LDH-N compared with 21.4-77.8% of patients aged >65 years. A higher proportion of older treatment-experienced patients (57.1‒77.8%) achieved LDH-N compared with older treatment-naive patients (21.4‒50.0%) at 26 and 52 weeks. In patients aged ≤65 years in both studies, 63.7‒89.4% achieved TA. In the >65 years subgroup, 14.3‒50.0% of treatment-naive patients achieved TA whereas in study 302, 54.5‒72.7% of patients achieved TA. The number of BTH events was low, with no events reported in older patients to date. Hgb stabilization was consistent in the ≤65 year subgroup between the studies; a higher proportion of older patients in study 302 (45.5‒71.4%) achieved stabilized Hgb compared with older patients in study 301 (14.3‒35.3%). A clinically significant 3-point change was seen in FACIT-fatigue scores (indicating improvements in fatigue), with higher scores observed for ravulizumab in both subgroups (Figure 1). One patient discontinued the extension of study 301 due to lung cancer onset during the 26-week period and died following discontinuation. Headache was the most frequent TEAE. The incidence of TEAEs reported during ravulizumab treatment up to 52 weeks did not increase vs the 26-week period, with few events (Table 3) and no difference between subgroups.
Conclusions: We present clinical outcomes in the largest cohort of patients with PNH (>65 years) on ravulizumab in a clinical trial setting to date. Ravulizumab was associated with similar efficacy and safety in both age subgroups and showed consistent and durable efficacy through 52 weeks of treatment. A higher proportion of patients in study 302 achieved all efficacy endpoints than in study 301, which can be due to patients' prior complement inhibitor experience. This observation was more evident in older patients. There were no BTH events in the older patients to date, and the number of infections in both subgroups was low. Ravulizumab was well tolerated in older patients with no additional safety concerns compared to younger patients.
Peffault De Latour:Apellis: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Alexion Pharmaceuticals Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Szer:Pfizer: Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Apellis: Consultancy; Alexion Pharmaceuticals Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Prevail Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Speakers Bureau. Kulasekararaj:Alexion Pharmaceuticals Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kim:Alexion Pharmaceuticals Inc.: Honoraria, Research Funding. Piatek:Alexion Pharmaceuticals: Consultancy, Research Funding. Kulagin:Alexion Pharmaceuticals Inc.: Consultancy, Research Funding. Hill:Alexion Pharmaceuticals Inc.: Current Employment. Wang:Alexion Pharmaceuticals Inc.: Current Employment. Yu:Alexion Pharmaceuticals Inc.: Current Employment. Ogawa:Alexion Pharmaceuticals Inc.: Current Employment. Schrezenmeier:Alexion Pharmaceuticals Inc.: Honoraria, Research Funding. Lee:Alexion Pharmaceuticals Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal